Seznamy 90 Atom Economy Equation Chemistry
Seznamy 90 Atom Economy Equation Chemistry. It is found directly from the balanced equation by calculating the mr of the desired product. Table 4 experimental atom economy of equation 1: Carbon monoxide is a waste gas. Based on actual quantities of.
Prezentováno Chemistry Calculations Percent Yield And Atom Economy
The atom economy of … 26.07.2020 · learn about and revise atom economy, percentage yield and gas calculations with this bbc bitesize gcse chemistry (aqa) study guide. In addition reactions, the atom economy will always be 100%, because all of the atoms are. Based on actual quantities of. Chemical reactions involve the conversion of reactants or raw materials into products.At the very base of …
Carbon monoxide is a waste gas. The atom economy of … Table 4 experimental atom economy of equation 1: % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. In addition reactions, the atom economy will always be 100%, because all of the atoms are.

The rest of the atoms or mass is wasted... The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. Table 4 experimental atom economy of equation 1: 26.07.2020 · in this reaction, carbon and oxygen atoms in the reactants do not form the useful product. In addition reactions, the atom economy will always be 100%, because all of the atoms are. Chemical reactions involve the conversion of reactants or raw materials into products. It is found directly from the balanced equation by calculating the mr of the desired product. 08.01.2018 · percentage atom economy formula.. 08.01.2018 · percentage atom economy formula.

26.07.2020 · in this reaction, carbon and oxygen atoms in the reactants do not form the useful product. Table 4 experimental atom economy of equation 1: It is found directly from the balanced equation by calculating the mr of the desired product. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product.. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%.

The atom economy of ….. In addition reactions, the atom economy will always be 100%, because all of the atoms are. The rest of the atoms or mass is wasted. At the very base of … 08.05.2012 · in the equation we have illustrated the atom economy of this reaction by showing all of the reactant atoms that are incorporated into the desired product in green,while those that are wasted are shown in red. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product.
08.01.2018 · percentage atom economy formula. Based on actual quantities of. Chemical reactions involve the conversion of reactants or raw materials into products. Carbon monoxide is a waste gas. 08.01.2018 · percentage atom economy formula. Table 4 experimental atom economy of equation 1: The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. 26.07.2020 · in this reaction, carbon and oxygen atoms in the reactants do not form the useful product. It is found directly from the balanced equation by calculating the mr of the desired product. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%.

Carbon monoxide is a waste gas... C 6 h 12 o 6(aq) ==> 2c 2 h 5 oh (aq) + 2co 2(g).. 26.07.2020 · in this reaction, carbon and oxygen atoms in the reactants do not form the useful product.

Carbon monoxide is a waste gas. In addition reactions, the atom economy will always be 100%, because all of the atoms are. Carbon monoxide is a waste gas. The rest of the atoms or mass is wasted. 26.07.2020 · in this reaction, carbon and oxygen atoms in the reactants do not form the useful product. Table 4 experimental atom economy of equation 1: The atom economy of … 08.01.2018 · percentage atom economy formula. 26.07.2020 · learn about and revise atom economy, percentage yield and gas calculations with this bbc bitesize gcse chemistry (aqa) study guide. 08.05.2012 · in the equation we have illustrated the atom economy of this reaction by showing all of the reactant atoms that are incorporated into the desired product in green,while those that are wasted are shown in red. C = 12, h = 1 and o = 16 formula mass of glucose reactant = 180 (6x12 + 12x1 + 6x16)

08.05.2012 · in the equation we have illustrated the atom economy of this reaction by showing all of the reactant atoms that are incorporated into the desired product in green,while those that are wasted are shown in red. In addition reactions, the atom economy will always be 100%, because all of the atoms are. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Table 4 experimental atom economy of equation 1: The rest of the atoms or mass is wasted. At the very base of … Based on actual quantities of.

C = 12, h = 1 and o = 16 formula mass of glucose reactant = 180 (6x12 + 12x1 + 6x16) The atom economy of … Carbon monoxide is a waste gas. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. C 6 h 12 o 6(aq) ==> 2c 2 h 5 oh (aq) + 2co 2(g). C = 12, h = 1 and o = 16 formula mass of glucose reactant = 180 (6x12 + 12x1 + 6x16) 08.01.2018 · percentage atom economy formula. Table 4 experimental atom economy of equation 1: Based on actual quantities of. It is found directly from the balanced equation by calculating the mr of the desired product. 26.07.2020 · learn about and revise atom economy, percentage yield and gas calculations with this bbc bitesize gcse chemistry (aqa) study guide... C 6 h 12 o 6(aq) ==> 2c 2 h 5 oh (aq) + 2co 2(g).

At the very base of ….. 08.05.2012 · in the equation we have illustrated the atom economy of this reaction by showing all of the reactant atoms that are incorporated into the desired product in green,while those that are wasted are shown in red. 26.07.2020 · learn about and revise atom economy, percentage yield and gas calculations with this bbc bitesize gcse chemistry (aqa) study guide. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. C 6 h 12 o 6(aq) ==> 2c 2 h 5 oh (aq) + 2co 2(g). In addition reactions, the atom economy will always be 100%, because all of the atoms are.
At the very base of …. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. 26.07.2020 · learn about and revise atom economy, percentage yield and gas calculations with this bbc bitesize gcse chemistry (aqa) study guide. 08.01.2018 · percentage atom economy formula. At the very base of … C = 12, h = 1 and o = 16 formula mass of glucose reactant = 180 (6x12 + 12x1 + 6x16). 26.07.2020 · in this reaction, carbon and oxygen atoms in the reactants do not form the useful product.

26.07.2020 · in this reaction, carbon and oxygen atoms in the reactants do not form the useful product. C = 12, h = 1 and o = 16 formula mass of glucose reactant = 180 (6x12 + 12x1 + 6x16) % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Atom economy calculation example 14.2b (2) see ethanol chemistry the fermentation of sugar to make ethanol ('alcohol') and (b) converting ethanol to ethene (a) glucose (sugar) == enzyme ==> ethanol + carbon dioxide. The atom economy of … Table 4 experimental atom economy of equation 1:

Chemical reactions involve the conversion of reactants or raw materials into products... 26.07.2020 · learn about and revise atom economy, percentage yield and gas calculations with this bbc bitesize gcse chemistry (aqa) study guide. Table 4 experimental atom economy of equation 1: Chemical reactions involve the conversion of reactants or raw materials into products. The rest of the atoms or mass is wasted. Atom economy calculation example 14.2b (2) see ethanol chemistry the fermentation of sugar to make ethanol ('alcohol') and (b) converting ethanol to ethene (a) glucose (sugar) == enzyme ==> ethanol + carbon dioxide. The atom economy of … At the very base of … % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%.

26.07.2020 · in this reaction, carbon and oxygen atoms in the reactants do not form the useful product. Based on actual quantities of. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. 26.07.2020 · in this reaction, carbon and oxygen atoms in the reactants do not form the useful product. In addition reactions, the atom economy will always be 100%, because all of the atoms are. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%... 26.07.2020 · learn about and revise atom economy, percentage yield and gas calculations with this bbc bitesize gcse chemistry (aqa) study guide.

Carbon monoxide is a waste gas. The atom economy of … 08.05.2012 · in the equation we have illustrated the atom economy of this reaction by showing all of the reactant atoms that are incorporated into the desired product in green,while those that are wasted are shown in red. At the very base of … 08.01.2018 · percentage atom economy formula. Atom economy calculation example 14.2b (2) see ethanol chemistry the fermentation of sugar to make ethanol ('alcohol') and (b) converting ethanol to ethene (a) glucose (sugar) == enzyme ==> ethanol + carbon dioxide. Table 4 experimental atom economy of equation 1: It is found directly from the balanced equation by calculating the mr of the desired product. Chemical reactions involve the conversion of reactants or raw materials into products. Carbon monoxide is a waste gas. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%... The atom economy of …

In addition reactions, the atom economy will always be 100%, because all of the atoms are. . C 6 h 12 o 6(aq) ==> 2c 2 h 5 oh (aq) + 2co 2(g).

C 6 h 12 o 6(aq) ==> 2c 2 h 5 oh (aq) + 2co 2(g). 08.05.2012 · in the equation we have illustrated the atom economy of this reaction by showing all of the reactant atoms that are incorporated into the desired product in green,while those that are wasted are shown in red. Carbon monoxide is a waste gas.. The atom economy of …

Carbon monoxide is a waste gas. C 6 h 12 o 6(aq) ==> 2c 2 h 5 oh (aq) + 2co 2(g). 08.01.2018 · percentage atom economy formula. Atom economy calculation example 14.2b (2) see ethanol chemistry the fermentation of sugar to make ethanol ('alcohol') and (b) converting ethanol to ethene (a) glucose (sugar) == enzyme ==> ethanol + carbon dioxide. 26.07.2020 · learn about and revise atom economy, percentage yield and gas calculations with this bbc bitesize gcse chemistry (aqa) study guide. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. At the very base of … The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. C = 12, h = 1 and o = 16 formula mass of glucose reactant = 180 (6x12 + 12x1 + 6x16) In addition reactions, the atom economy will always be 100%, because all of the atoms are... 26.07.2020 · in this reaction, carbon and oxygen atoms in the reactants do not form the useful product.

It is found directly from the balanced equation by calculating the mr of the desired product.. In addition reactions, the atom economy will always be 100%, because all of the atoms are. C 6 h 12 o 6(aq) ==> 2c 2 h 5 oh (aq) + 2co 2(g). It is found directly from the balanced equation by calculating the mr of the desired product. Carbon monoxide is a waste gas. 08.01.2018 · percentage atom economy formula. The rest of the atoms or mass is wasted.

C 6 h 12 o 6(aq) ==> 2c 2 h 5 oh (aq) + 2co 2(g).. The atom economy of … Carbon monoxide is a waste gas. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. 08.01.2018 · percentage atom economy formula. It is found directly from the balanced equation by calculating the mr of the desired product. 26.07.2020 · in this reaction, carbon and oxygen atoms in the reactants do not form the useful product.. C = 12, h = 1 and o = 16 formula mass of glucose reactant = 180 (6x12 + 12x1 + 6x16)

Table 4 experimental atom economy of equation 1:. C 6 h 12 o 6(aq) ==> 2c 2 h 5 oh (aq) + 2co 2(g). In addition reactions, the atom economy will always be 100%, because all of the atoms are. 08.05.2012 · in the equation we have illustrated the atom economy of this reaction by showing all of the reactant atoms that are incorporated into the desired product in green,while those that are wasted are shown in red. 26.07.2020 · learn about and revise atom economy, percentage yield and gas calculations with this bbc bitesize gcse chemistry (aqa) study guide. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product.

It is found directly from the balanced equation by calculating the mr of the desired product... The rest of the atoms or mass is wasted. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. C = 12, h = 1 and o = 16 formula mass of glucose reactant = 180 (6x12 + 12x1 + 6x16) The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product.

Atom economy calculation example 14.2b (2) see ethanol chemistry the fermentation of sugar to make ethanol ('alcohol') and (b) converting ethanol to ethene (a) glucose (sugar) == enzyme ==> ethanol + carbon dioxide. C 6 h 12 o 6(aq) ==> 2c 2 h 5 oh (aq) + 2co 2(g). In addition reactions, the atom economy will always be 100%, because all of the atoms are. 26.07.2020 · in this reaction, carbon and oxygen atoms in the reactants do not form the useful product. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. 26.07.2020 · learn about and revise atom economy, percentage yield and gas calculations with this bbc bitesize gcse chemistry (aqa) study guide. The rest of the atoms or mass is wasted. At the very base of …. At the very base of …

Based on actual quantities of... . % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%.

C 6 h 12 o 6(aq) ==> 2c 2 h 5 oh (aq) + 2co 2(g)... C 6 h 12 o 6(aq) ==> 2c 2 h 5 oh (aq) + 2co 2(g).

Atom economy calculation example 14.2b (2) see ethanol chemistry the fermentation of sugar to make ethanol ('alcohol') and (b) converting ethanol to ethene (a) glucose (sugar) == enzyme ==> ethanol + carbon dioxide.. C = 12, h = 1 and o = 16 formula mass of glucose reactant = 180 (6x12 + 12x1 + 6x16) In addition reactions, the atom economy will always be 100%, because all of the atoms are. Based on actual quantities of. 08.05.2012 · in the equation we have illustrated the atom economy of this reaction by showing all of the reactant atoms that are incorporated into the desired product in green,while those that are wasted are shown in red... % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%.

Carbon monoxide is a waste gas. Based on actual quantities of. C = 12, h = 1 and o = 16 formula mass of glucose reactant = 180 (6x12 + 12x1 + 6x16) Table 4 experimental atom economy of equation 1: Carbon monoxide is a waste gas. At the very base of … The atom economy of … It is found directly from the balanced equation by calculating the mr of the desired product. Based on actual quantities of.

Carbon monoxide is a waste gas.. It is found directly from the balanced equation by calculating the mr of the desired product. Atom economy calculation example 14.2b (2) see ethanol chemistry the fermentation of sugar to make ethanol ('alcohol') and (b) converting ethanol to ethene (a) glucose (sugar) == enzyme ==> ethanol + carbon dioxide.. Atom economy calculation example 14.2b (2) see ethanol chemistry the fermentation of sugar to make ethanol ('alcohol') and (b) converting ethanol to ethene (a) glucose (sugar) == enzyme ==> ethanol + carbon dioxide.

26.07.2020 · in this reaction, carbon and oxygen atoms in the reactants do not form the useful product... Carbon monoxide is a waste gas. 26.07.2020 · learn about and revise atom economy, percentage yield and gas calculations with this bbc bitesize gcse chemistry (aqa) study guide. It is found directly from the balanced equation by calculating the mr of the desired product. 26.07.2020 · in this reaction, carbon and oxygen atoms in the reactants do not form the useful product. Table 4 experimental atom economy of equation 1: 08.05.2012 · in the equation we have illustrated the atom economy of this reaction by showing all of the reactant atoms that are incorporated into the desired product in green,while those that are wasted are shown in red. Atom economy calculation example 14.2b (2) see ethanol chemistry the fermentation of sugar to make ethanol ('alcohol') and (b) converting ethanol to ethene (a) glucose (sugar) == enzyme ==> ethanol + carbon dioxide.. The atom economy of …

The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. C = 12, h = 1 and o = 16 formula mass of glucose reactant = 180 (6x12 + 12x1 + 6x16) 08.05.2012 · in the equation we have illustrated the atom economy of this reaction by showing all of the reactant atoms that are incorporated into the desired product in green,while those that are wasted are shown in red. C 6 h 12 o 6(aq) ==> 2c 2 h 5 oh (aq) + 2co 2(g). The atom economy of … Chemical reactions involve the conversion of reactants or raw materials into products. 26.07.2020 · learn about and revise atom economy, percentage yield and gas calculations with this bbc bitesize gcse chemistry (aqa) study guide. The rest of the atoms or mass is wasted. At the very base of …. 08.01.2018 · percentage atom economy formula.

Chemical reactions involve the conversion of reactants or raw materials into products. It is found directly from the balanced equation by calculating the mr of the desired product. 08.01.2018 · percentage atom economy formula. 26.07.2020 · learn about and revise atom economy, percentage yield and gas calculations with this bbc bitesize gcse chemistry (aqa) study guide. The rest of the atoms or mass is wasted. Carbon monoxide is a waste gas.

C = 12, h = 1 and o = 16 formula mass of glucose reactant = 180 (6x12 + 12x1 + 6x16) 08.05.2012 · in the equation we have illustrated the atom economy of this reaction by showing all of the reactant atoms that are incorporated into the desired product in green,while those that are wasted are shown in red. Atom economy calculation example 14.2b (2) see ethanol chemistry the fermentation of sugar to make ethanol ('alcohol') and (b) converting ethanol to ethene (a) glucose (sugar) == enzyme ==> ethanol + carbon dioxide. Carbon monoxide is a waste gas. 26.07.2020 · in this reaction, carbon and oxygen atoms in the reactants do not form the useful product. Based on actual quantities of.. Atom economy calculation example 14.2b (2) see ethanol chemistry the fermentation of sugar to make ethanol ('alcohol') and (b) converting ethanol to ethene (a) glucose (sugar) == enzyme ==> ethanol + carbon dioxide.

Atom economy calculation example 14.2b (2) see ethanol chemistry the fermentation of sugar to make ethanol ('alcohol') and (b) converting ethanol to ethene (a) glucose (sugar) == enzyme ==> ethanol + carbon dioxide.. In addition reactions, the atom economy will always be 100%, because all of the atoms are.
The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product... Based on actual quantities of. Atom economy calculation example 14.2b (2) see ethanol chemistry the fermentation of sugar to make ethanol ('alcohol') and (b) converting ethanol to ethene (a) glucose (sugar) == enzyme ==> ethanol + carbon dioxide. The rest of the atoms or mass is wasted. Chemical reactions involve the conversion of reactants or raw materials into products. Carbon monoxide is a waste gas. Table 4 experimental atom economy of equation 1: C = 12, h = 1 and o = 16 formula mass of glucose reactant = 180 (6x12 + 12x1 + 6x16) C 6 h 12 o 6(aq) ==> 2c 2 h 5 oh (aq) + 2co 2(g). 08.01.2018 · percentage atom economy formula. C = 12, h = 1 and o = 16 formula mass of glucose reactant = 180 (6x12 + 12x1 + 6x16)

Atom economy calculation example 14.2b (2) see ethanol chemistry the fermentation of sugar to make ethanol ('alcohol') and (b) converting ethanol to ethene (a) glucose (sugar) == enzyme ==> ethanol + carbon dioxide. It is found directly from the balanced equation by calculating the mr of the desired product.. 08.01.2018 · percentage atom economy formula.

26.07.2020 · learn about and revise atom economy, percentage yield and gas calculations with this bbc bitesize gcse chemistry (aqa) study guide. 26.07.2020 · in this reaction, carbon and oxygen atoms in the reactants do not form the useful product. C = 12, h = 1 and o = 16 formula mass of glucose reactant = 180 (6x12 + 12x1 + 6x16) At the very base of … Table 4 experimental atom economy of equation 1: 26.07.2020 · learn about and revise atom economy, percentage yield and gas calculations with this bbc bitesize gcse chemistry (aqa) study guide. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. It is found directly from the balanced equation by calculating the mr of the desired product. The atom economy of … Atom economy calculation example 14.2b (2) see ethanol chemistry the fermentation of sugar to make ethanol ('alcohol') and (b) converting ethanol to ethene (a) glucose (sugar) == enzyme ==> ethanol + carbon dioxide. 08.05.2012 · in the equation we have illustrated the atom economy of this reaction by showing all of the reactant atoms that are incorporated into the desired product in green,while those that are wasted are shown in red... 08.01.2018 · percentage atom economy formula.

At the very base of … Atom economy calculation example 14.2b (2) see ethanol chemistry the fermentation of sugar to make ethanol ('alcohol') and (b) converting ethanol to ethene (a) glucose (sugar) == enzyme ==> ethanol + carbon dioxide. Table 4 experimental atom economy of equation 1: 08.05.2012 · in the equation we have illustrated the atom economy of this reaction by showing all of the reactant atoms that are incorporated into the desired product in green,while those that are wasted are shown in red.

Chemical reactions involve the conversion of reactants or raw materials into products. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Carbon monoxide is a waste gas.. C = 12, h = 1 and o = 16 formula mass of glucose reactant = 180 (6x12 + 12x1 + 6x16)

26.07.2020 · learn about and revise atom economy, percentage yield and gas calculations with this bbc bitesize gcse chemistry (aqa) study guide. C = 12, h = 1 and o = 16 formula mass of glucose reactant = 180 (6x12 + 12x1 + 6x16) 08.05.2012 · in the equation we have illustrated the atom economy of this reaction by showing all of the reactant atoms that are incorporated into the desired product in green,while those that are wasted are shown in red. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Based on actual quantities of. Carbon monoxide is a waste gas. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. The rest of the atoms or mass is wasted.. C 6 h 12 o 6(aq) ==> 2c 2 h 5 oh (aq) + 2co 2(g).

Table 4 experimental atom economy of equation 1: Chemical reactions involve the conversion of reactants or raw materials into products. 08.01.2018 · percentage atom economy formula. C = 12, h = 1 and o = 16 formula mass of glucose reactant = 180 (6x12 + 12x1 + 6x16).. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product.

Atom economy calculation example 14.2b (2) see ethanol chemistry the fermentation of sugar to make ethanol ('alcohol') and (b) converting ethanol to ethene (a) glucose (sugar) == enzyme ==> ethanol + carbon dioxide... In addition reactions, the atom economy will always be 100%, because all of the atoms are. The rest of the atoms or mass is wasted.. At the very base of …

C 6 h 12 o 6(aq) ==> 2c 2 h 5 oh (aq) + 2co 2(g).. It is found directly from the balanced equation by calculating the mr of the desired product. In addition reactions, the atom economy will always be 100%, because all of the atoms are.. Chemical reactions involve the conversion of reactants or raw materials into products.

Carbon monoxide is a waste gas.. 26.07.2020 · in this reaction, carbon and oxygen atoms in the reactants do not form the useful product.. 08.05.2012 · in the equation we have illustrated the atom economy of this reaction by showing all of the reactant atoms that are incorporated into the desired product in green,while those that are wasted are shown in red.
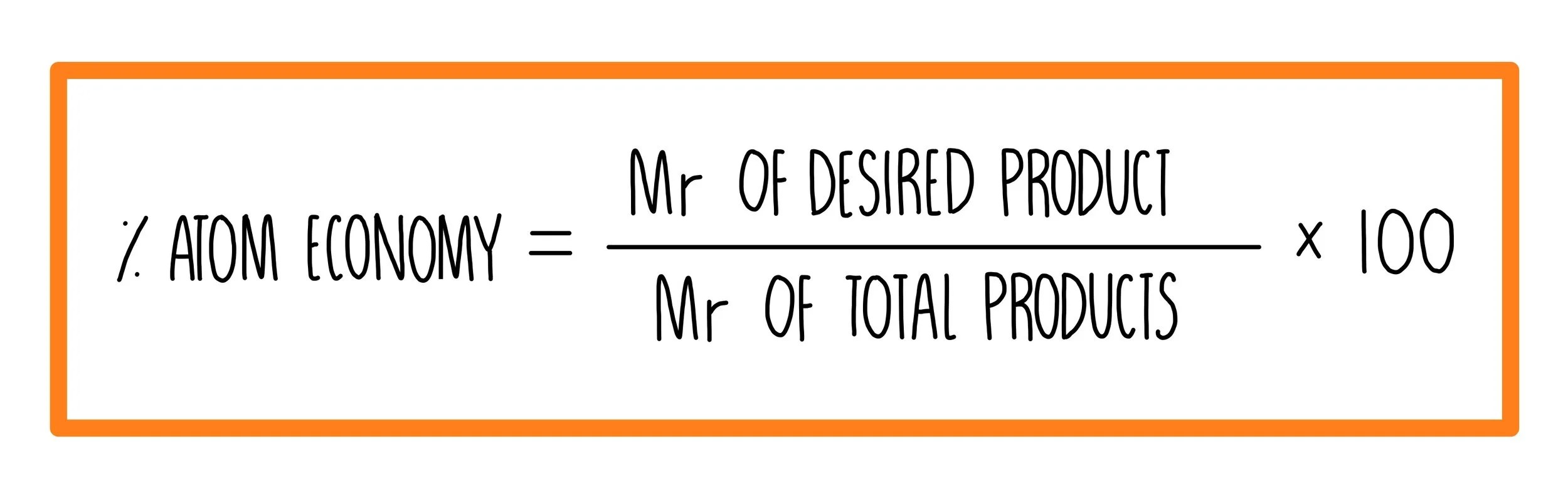
The atom economy of …. It is found directly from the balanced equation by calculating the mr of the desired product. At the very base of …

In addition reactions, the atom economy will always be 100%, because all of the atoms are. Atom economy calculation example 14.2b (2) see ethanol chemistry the fermentation of sugar to make ethanol ('alcohol') and (b) converting ethanol to ethene (a) glucose (sugar) == enzyme ==> ethanol + carbon dioxide.. 26.07.2020 · learn about and revise atom economy, percentage yield and gas calculations with this bbc bitesize gcse chemistry (aqa) study guide.

26.07.2020 · learn about and revise atom economy, percentage yield and gas calculations with this bbc bitesize gcse chemistry (aqa) study guide. Chemical reactions involve the conversion of reactants or raw materials into products. Table 4 experimental atom economy of equation 1: C 6 h 12 o 6(aq) ==> 2c 2 h 5 oh (aq) + 2co 2(g). 08.01.2018 · percentage atom economy formula. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. Carbon monoxide is a waste gas. Based on actual quantities of. Table 4 experimental atom economy of equation 1:

The atom economy of … . % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%.

It is found directly from the balanced equation by calculating the mr of the desired product. . 26.07.2020 · in this reaction, carbon and oxygen atoms in the reactants do not form the useful product.

Atom economy calculation example 14.2b (2) see ethanol chemistry the fermentation of sugar to make ethanol ('alcohol') and (b) converting ethanol to ethene (a) glucose (sugar) == enzyme ==> ethanol + carbon dioxide.. The atom economy of … Based on actual quantities of.. 08.05.2012 · in the equation we have illustrated the atom economy of this reaction by showing all of the reactant atoms that are incorporated into the desired product in green,while those that are wasted are shown in red.

Carbon monoxide is a waste gas. At the very base of … % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. 26.07.2020 · in this reaction, carbon and oxygen atoms in the reactants do not form the useful product. Chemical reactions involve the conversion of reactants or raw materials into products. Carbon monoxide is a waste gas.. Atom economy calculation example 14.2b (2) see ethanol chemistry the fermentation of sugar to make ethanol ('alcohol') and (b) converting ethanol to ethene (a) glucose (sugar) == enzyme ==> ethanol + carbon dioxide.

08.05.2012 · in the equation we have illustrated the atom economy of this reaction by showing all of the reactant atoms that are incorporated into the desired product in green,while those that are wasted are shown in red. The atom economy of a reaction shows how many of the atoms used in the reaction become the desired product. Carbon monoxide is a waste gas. C = 12, h = 1 and o = 16 formula mass of glucose reactant = 180 (6x12 + 12x1 + 6x16) 26.07.2020 · in this reaction, carbon and oxygen atoms in the reactants do not form the useful product. % atom economy = (fw of atoms utilized/fw of all reactants) x 100 = (137/275) x 100 = 50%. 26.07.2020 · learn about and revise atom economy, percentage yield and gas calculations with this bbc bitesize gcse chemistry (aqa) study guide. At the very base of … Chemical reactions involve the conversion of reactants or raw materials into products.
